Cells
Fusion
There are several known methods how to fuse
living cells. The first one uses immersion of cells
into
chemical solution (e.g. 50% PEG 1500)1, the
second method employs
external electric field for the cell perforation2.
Unfortunately
both these methods cannot be easily used
to fuse ndividually
selected cells. The third method of cell fusion uses focused laser
beams that
evaporate tiny volume of the cell membrane3, 4.
Laser induced cells
fusion takes place under an objective of a microscope and easily
enables the
study of the fusion dynamics.
The
principle of the laser-induced cell fusion:
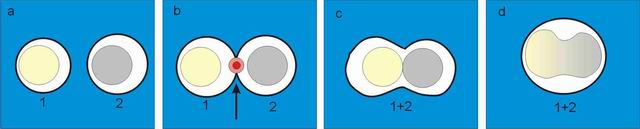
Two selected
cells (No. 1 and 2) are brought into contact by optical tweezers
(see
subfigure a). Bigger cells, which cannot be dragged
by
optical tweezers, are
transported by mechanical micromanipulator (Eppendorf TransferManâ NK) and
micropipette (Eppendorf CellTram
Air). A sequence of pulses is applied at the point of cells contact
(subfigure b
denoted by arrow), the membranes in contact are perforated and the
content of
both cells is mixed (subfigure c). A few minutes
later the fusion
product takes round shape again and cell nuclei start to mix.
Experiment:
The experiments were performed in cooparation
with the group of Prof.
S. Kozubek from Institute of Biophyscis ASCR, Prof. M. Kozubek
from Faculty of Informatics Masaryk University in Brno. The first
experiment with cells fusion we performed with human lymphocyte cells
HL60. Later on we worked with adherent MCF 7 cells that fused easier.
They were placed on the micro-grid
cover slip
(CELLocate, square size 55 mm) for
easier localization under the
microscope.
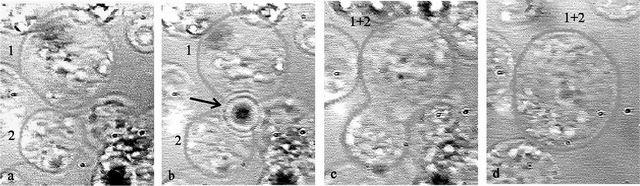
Figure shows
the employment of the trapping and
cutting beam for the cell fusion HL60. Two human lymphocyte cells (No.
1 and 2)
were brought into touch by optical tweezers (see subfigure
a). Laser pulses of the cutting beam
(dark spot in subfigure b denoted
by
the arrow) perforated the outer cell membrane and both cells fused
together (c, d). Subfigure c is taken 40s and subfigure d
160s after the wall perforation.
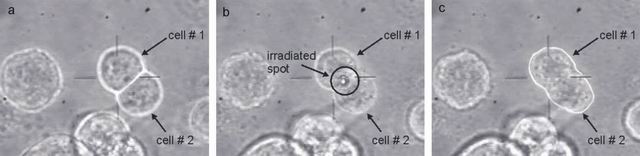
Two MCF 7 cells (denoted as 1, 2) form a cluster (see subfigure a). Laser pulses are applied to perforate the cell membrane at the point of contact (light spot in subfigure b denoted by circle). The content of both cells is mixed (see subfigure c).
Further studies with fused cells
The cell nuclei were dyed by low flourochrome concentration for their easier identification in the fused cell. One of MCF7 cell was colored by Hoechst 33342 which provides blue fluorescence and the other by Propidium Iodide which provides red fluorescence. Optical tweezers or micropipette was used to bring both cells to contact and series of 4-5 pulses from UV laser (an average energy per pulse was equal to 8 mJ) perforated the membrane. To achieve this, it was necessary to move the sample vertically so that the point of contact coincided with focal plane of the UV beam. The plasmatic membranes disrupted by thermal ablation and their ends immediately joined to form a single hybrid cell. After the fusion, the cells were cultured in a fresh medium. In the intervals of 4, 8, 12, and 24 hours fused cells were fixed by paraformaldehyde and afterwards they were studied by fluorescence in situ hybridization using the specific DNA probe for chromosomes 12 and 7 centromere. We found out that non-fused single MCF7 cells had three signals corresponding to the chromosomes 12 and 7 (trisomia of chromosomes 12 and 7), meanwhile six signals were found in fused cells (see the figures below). Using high-resolution cytomery5, the dynamics of the chromosome arrangement in the progress of time after the cell fusion was studied. We observed that the homologous chromosomes in the fused cells do not merge together but occupy their separate positions in the fused nucleus.

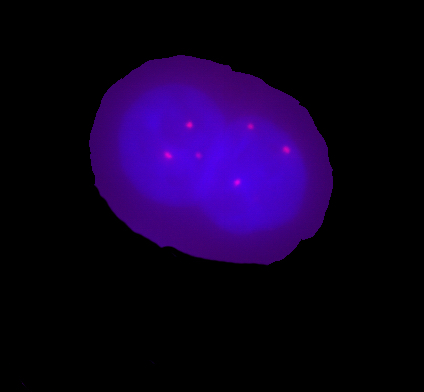
Unfortunately we have not observed division of fused cell even if we observed it for several days. Moreover they usually died within this period.
Further reading:
J.
Jezek, S. Palsa, E. Lukasova,
S. Kozubek, P. Jakl, M. Sery, A. Jonas, M. Liska, P. Zemanek: "Employment
of laser induced fusion of living cells for the study of spatial
structure of chromatin",
Proceedings of
SPIE 5259, 336-340, 2003.
J.
Jezek, S. Palsa, E. Lukasova,
S. Kozubek, P. Jakl, M. Sery, A. Jonas, M. Liska, P. Zemanek: "Spatial
structure of chromatin in hybrid cells produced by laser induced fusion
studied by optical microscopy",
Proceedings
of SPIE 5036, 630-634, 2002.
References:
1.
G. A. Neil
and U. Zimmermann, "Electrofusion", Methods Enzymol.
220,
pp. 174-196, 1993.
2.
A.
Strömberg
et al, " Manipulating the genetic identity and
biochemical surface
properties of individual cells with electric-field-induced fusion", PNAS
97 (1), pp. 7-11, 2000.
3.
S. Sato et
al,"Achievement of laser fusion of biological cells using UV
pulsed
dye laser beams", Appl. Phys. B 54,
pp. 531-533, 1992.
4.
R. Wiegand et
al, "Laser-induced fusion of cells and plant protoplasts", Journal
of Cell Science 88, pp. 145-149, 1987.
5.
M. Kozubek et
al, "Combined confocal and wide-field high-resolution
cytometry of
FISH-stained cells" Cytometry 45,
pp.1-12, 2001.
Send comments to webmaster
Last modification: 11 Jul 2007

