Biophotonics and Optofluidics
The primary goal of the Biophotonics and Optofluidics group is to monitor, characterize, identify and describe microorganisms and the process inside of such objects. The main activities of our research group can be divided to several sections:
Identification of microorganisms by Raman microspectroscopy
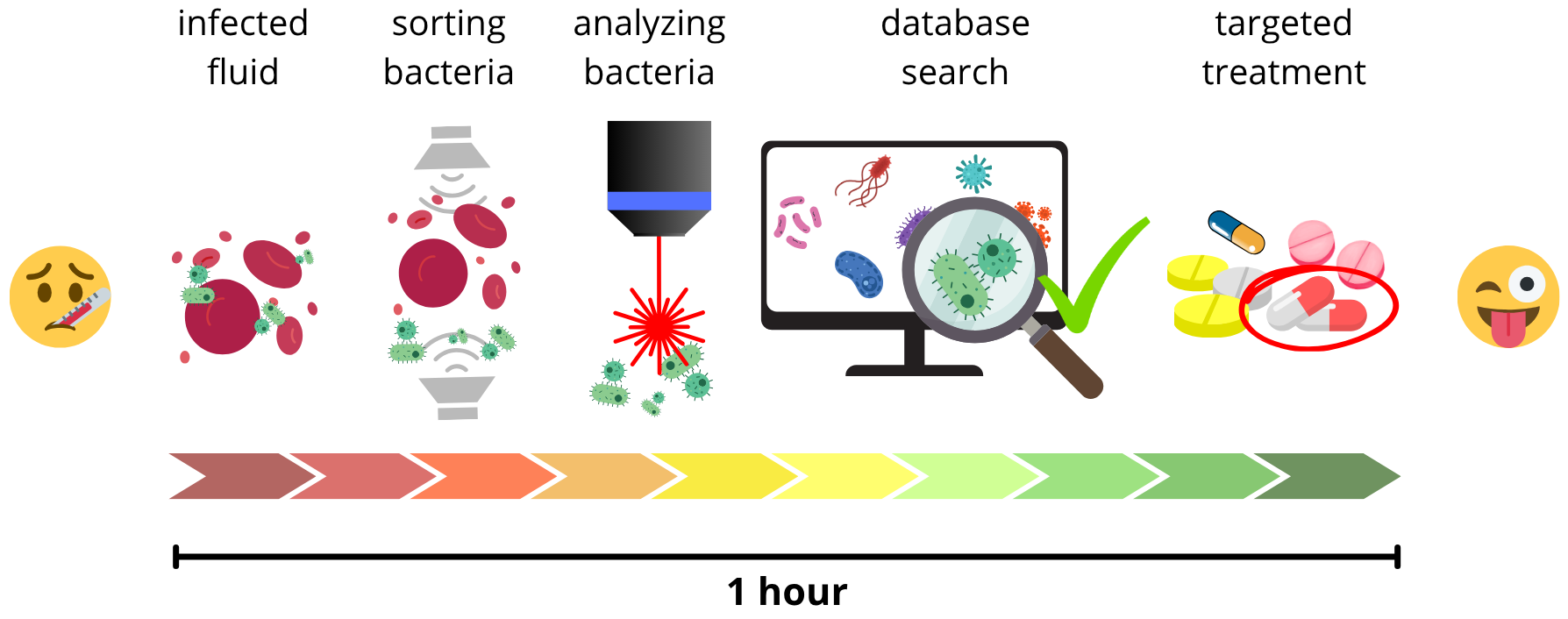
Identifying microorganisms from small samples quickly and reliably is crucial for diagnosing infections. Ideally, methods would need little preparation, allow automated analysis, and classify them rapidly utilizing a database. Current practices, however, often take days, delaying results and leading to inappropriate antibiotic use, worsening patient outcomes and contributing to bacterial resistance to the antibiotics.
Raman microspectroscopy offers a promising solution as the Raman scattered spectra reflect cellular components like nucleic acids, proteins, carbohydrates, and lipids, effectively creating a cellular fingerprint. This enables rapid differentiation of microorganisms, their physiological states, and phenotype changes. The goal is to create Raman setup applicable in clinics to successfully sort bacteria from blood or urine and identify them with Raman, all within one hour.
Thanks to the unique fingerprints, different bacterial strains can be distinguished. To support this, a large database of hundreds of strains has been developed, while machine learning algorithms can be employed to identify unknown bacteria by comparing their spectra with the stored data.
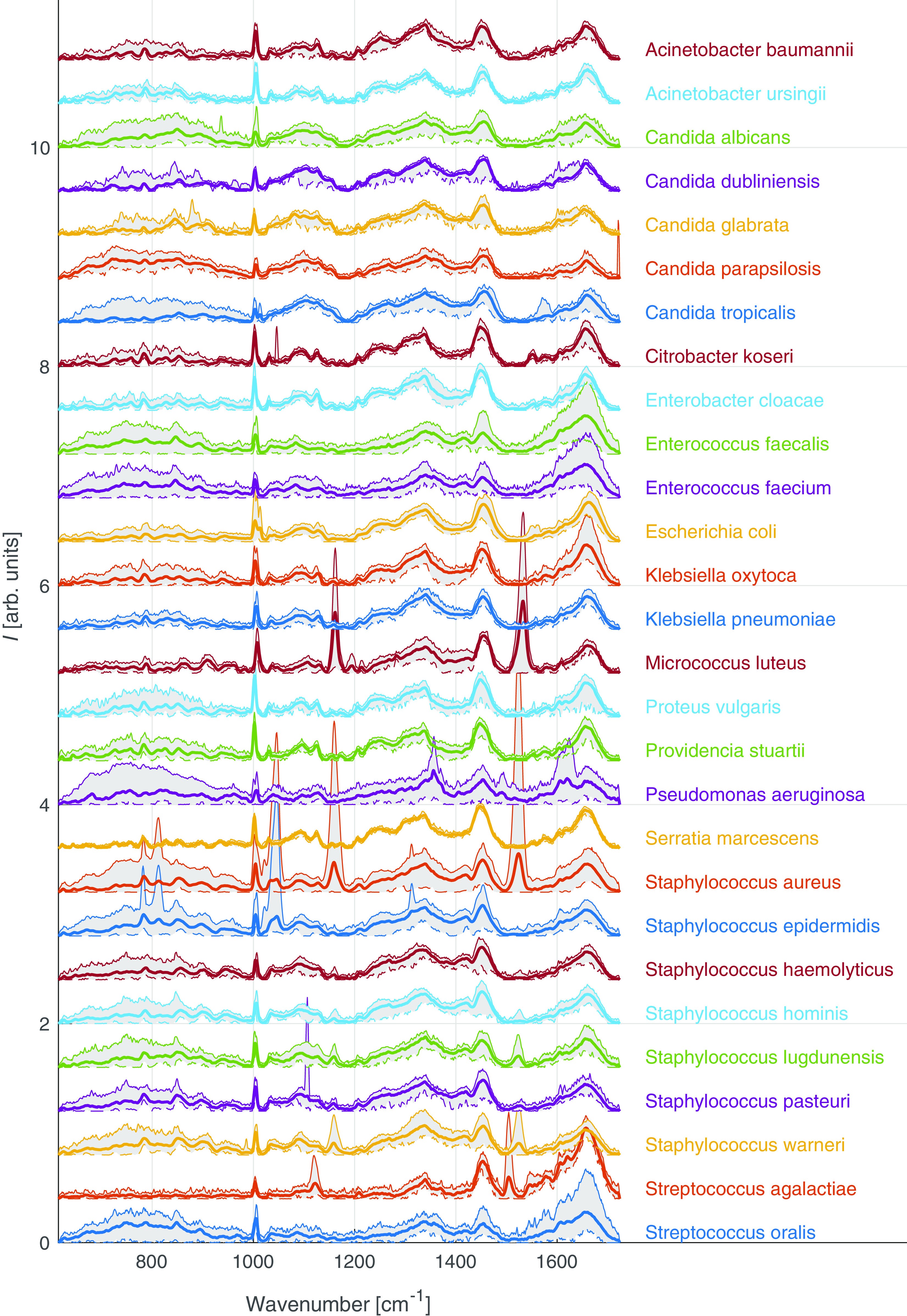

Fig. 2 – Examples of different Raman signals for different strains of bacteria (left). A confusion matrix with scores for bacteria identification (right).
Surface-enhanced Raman spectroscopy for monitoring of bacterial stress
Outside of just identifying the bacteria, surface-enhanced Raman (SERS) spectroscopy can be used to monitor the cells response to stress. Bacteria under stress produce chemicals, such as adenine, which is not observable with basic Raman, but is very sensitive for SERS. Analyses of adenine production could serve as a way to establish bacterial resistance to antibiotics.
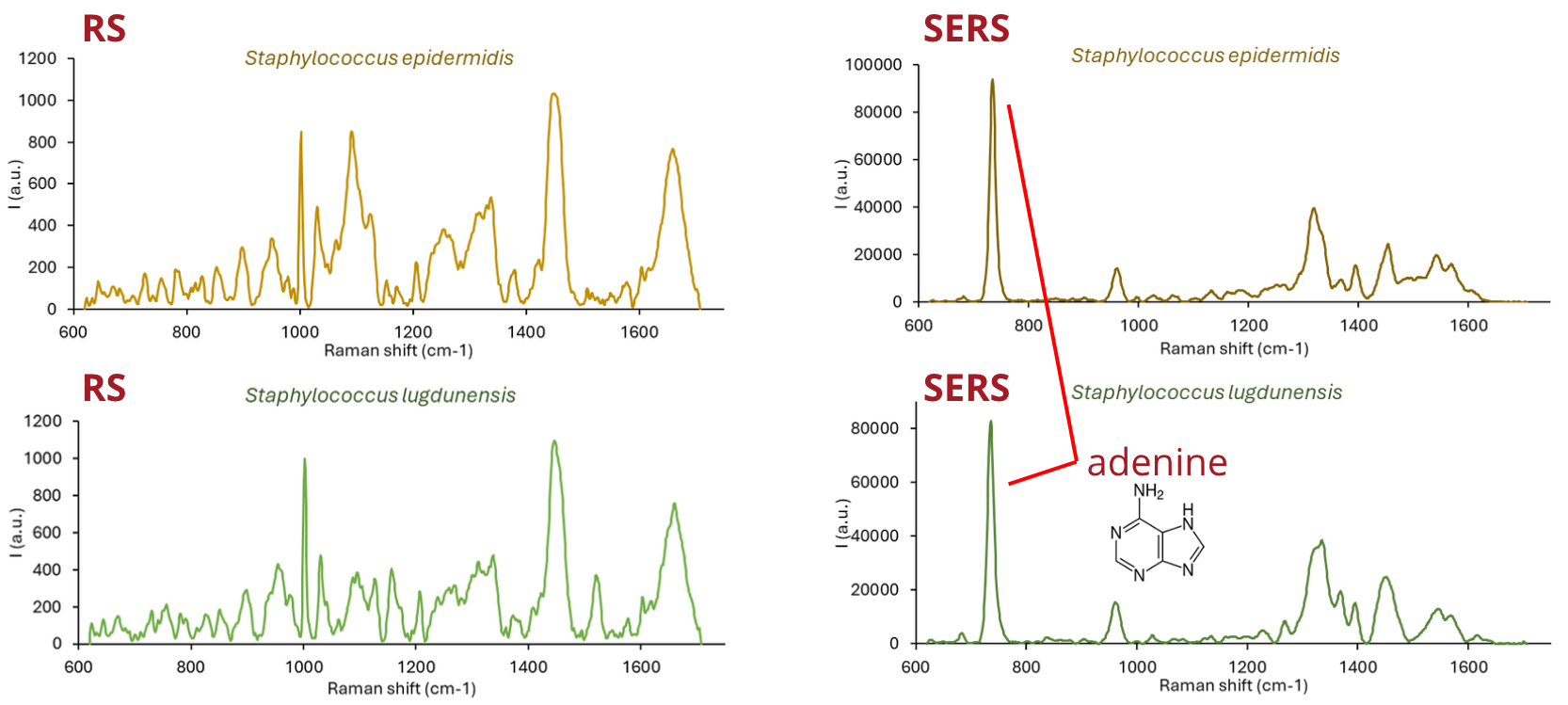
Fig. 3 – Basic Raman spectra of bacteria (left). SERS spectra of bacteria (right).
Microfluidic chips for trapping and focusing bacterial cells
The analyses of microorganisms often rely on microfluidic devices (usually PDMS chips), which allow precise handling of very small sample volumes. Several approaches are used for bacterial identification within microfluidics, such as acoustofluidic sorting or accumulation based on cell properties (size, weight).

Fig. 4 – Example of an acoustofluidic chip (left). Particles flowing without (middle) and with (right) standing acoustic wave (SAW).
Another approach is guiding cells through sawblade-like channels where applied voltage causes them to oscillate through the channel in both directions, allowing for repeated or prolonged analyses and further reducing the sample volume (“infinite channel”).
Fig. 5 – A prototype of the sawblade microfluidic platform with oscillating cells.
Raman tweezers for the detection of microplastics
The improper handling and disposal of plastics lead to their accumulation in landfills and environment. There plastics are exposed to weathering effects, such as mechanical abrasion, chemical degradation, or photodegradation, and form smaller fragments that are released to the environment. These fragments are called microplastics (MPs) (1 µm–5 mm) and nanoplastics (NPs) (<1 µm). Their ubiquitous presence raises concerns about their effect on human health. Just as Raman microspectroscopy is applicable for microorganisms, it also enables sensitive detection of MPs with high spatial resolution, especially when combined with optical tweezers. This combined technique is called Raman tweezers. Raman tweezers can be used to optically trap MPs and acquire chemical information about the particles.
Fig. 6 – A scheme of a degradation process to analysis of MPs with Raman tweezers (left). Raman spectra of a) all particles averaged, b) and c) different-sized particles, d) particle containing additional dye phthalocyanine (PC) (right).
Analysis of enzymatic degradation of chlorinated pollutants by high-power Raman microspectroscopy
Just as microplastics, chlorinated compounds also present an environmental threat. They belong to the group of persistent organic pollutants and are often considered (at least potential) carcinogens. Due to their persistency their enzymatic degradation to harmless compounds could be a crucial step in remediation of contaminated areas. The process is a cascade of five reactions catalyzed by three enzymes: haloalkane dehalogenase (DhaA), epoxid hydrolase (EchA), halohydrin dehalogenase (HheC). High-power Raman spectroscopy utilizes a high-power laser (6 W) for monitoring the degradation process and to establish the perfect enzyme ratio for the degradation. Specifically, the degradation of 1,2,3-trichloropropane (TCP) to glycerol (GLY) is examined.
Fig. 7 – The setup of the in-house built high-power Raman microspectrometer.
Fig. 8 – The degradation cascade (left). The dependence of the GLY production on the DhaA:EchA:HheC enzyme ratios (right).
Robotic Raman for plant analyses
Automated robotic system for detection of metabolic substances and pathogens in plants. The robotic arm with Raman probe is a part of multi-analytical system to explore both positive and negative influences on the plants growth.
Fig. 9 – The robotic arm with Raman probe.