Přednáška: Impact of selected physical factors on cell fate
Srdečně vás zveme na přednášku “Vliv vybraných fyzikálních faktorů na buněčné mechanismy”, kterou přednese dne 14.3.2024 v 10:00 v přednáškovém sálu ÚPT AV ČR, Oleg Lunov, Ph.D., z Fyzikálního ústavu AV ČR.
Přednáška se uskuteční za podpory projektu Strategie AV21, program „Průlomové technologie budoucnosti“, jehož koordinátorem je prof. Josef Lazar, ředitel Ústavu přístrojové techniky AV ČR.
Anotace k přednášce:
Department of Optical and Biophysical Systems, Institute of Physics of the Czech Academy of Sciences, Prague, 18221, Czech Republic
Physics has always played a pivotal role in advancing biological and medical research. Its significant contributions, spanning a wide range of applications, have evolved over time. As our understanding of the molecular mechanisms of diseases deepens, and with the aid of emerging technologies, the contributions of physics to healthcare are expected to expand further. Therefore, the Laboratory of Biophysics of Institute of Physics of the Czech Academy of Sciences merges physical and biological research approaches to create a scientific foundation for novel therapeutic strategies and interventions. Here we will discuss how selected physical factors affect cell functionality. We will focus on two main research lines of the Laboratory of Biophysics: nanomaterials-cell interactions and effects of physical forces on cellular behavior (Fig. 1.).
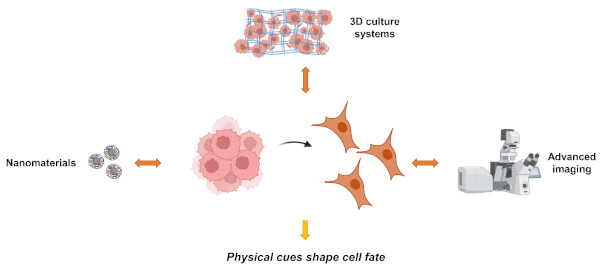
Fig. 1. Research directions of the Laboratory of Biophysics. Our goal is to gain deep insights in fundamental mechanistic understanding of how selected physical factors affect cellular machinery.
Within the nanomaterials-cell interactions we will discuss the problem of the adverse effects of nanomedicines. While some nanomedicines have been approved for use in the last 20 years, their journey from the laboratory bench to actual treatments has been slow. To make nanotechnology in medicine more successful, it's crucial to understand how nanoparticles might be harmful at the cellular and molecular levels. Therefore, we explored how nanoparticles might be causing trouble by disrupting lysosomes. We deeply investigated the adverse effects of nanomedicines and revealed that nanoparticle-induced dysregulation of lysosomes serves as the main reason for nanotoxicity.
The part of the effects physical stimuli of the cellular microenvironment on cells will be devoted to how mechanical cues regulate key cellular functions. Accumulating evidence suggests that tumor cells face variable mechanical stimuli which may induce metabolic rewiring of tumor cells. However, the knowledge of how tumor cells adapt metabolism to external mechanical cues is still limited. We, therefore, designed soft 3D collagen scaffolds mimicking pathological mechanical environment to decipher how liver tumor cells would adapt their metabolic activity to physical stimuli of the cellular microenvironment. Our data reveal coupling of liver tumor glycolysis to mechanical cues.
References
- Harrison SP, Siller R, Tanaka Y, Chollet ME, de la Morena-Barrio ME, Xiang Y, Patterson B, Andersen E, Bravo-Pérez C, Kempf H, Åsrud KS, Lunov O, Dejneka A, Mowinckel MC, Stavik B, Sandset PM, Melum E, Baumgarten S, Bonanini F, Kurek D, Mathapati S, Almaas R, Sharma K, Wilson SR, Skottvoll FS, Boger IC, Bogen IL, Nyman TA, Wu JJ, Bezrouk A, Cizkova D, Corral J, Mokry J, Zweigerdt R, Park IH, Sullivan GJ. Scalable production of tissue-like vascularized liver organoids from human PSCs. Exp. Mol. Med. 2023; 55: 2005-2024.
- Uzhytchak M, Lunova M, Smolková B, Jirsa M, Dejneka A, Lunov O. Iron oxide nanoparticles trigger endoplasmic reticulum damage in steatotic hepatic cells. Nanoscale Adv. 2023; 5: 4250.
- Uzhytchak M, Smolková B, Lunova M, Frtús A, Jirsa M, Dejneka A, Lunov O. Lysosomal nanotoxicity: Impact of nanomedicines on lysosomal function. Adv. Drug Deliv. Rev. 2023; 197: 114828.
- Frtús A, Smolková B, Uzhytchak M, Lunova M, Jirsa M, Petrenko Y, Dejneka A, Lunov O. Mechanical regulation of mitochondrial dynamics and function in a 3D-engineered liver tumor microenvironment. ACS Biomater. Sci. Eng. 2023; 9: 2408-2425.
- Frtús A, Smolková B, Uzhytchak M, Lunova M, Jirsa M, Henry SJW, Dejneka A, Stephanopoulos N, Lunov O. The interactions between DNA nanostructures and cells: A critical overview from a cell biology perspective. Acta Biomater. 2022; 146: 10-22.
- Smolková B, MacCulloch T, Rockwood TF, Liu M, Henry SJW, Frtús A, Uzhytchak M, Lunova M, Hof M, Jurkiewicz P, Dejneka A, Stephanopoulos N, Lunov O. Protein corona inhibits endosomal escape of functionalized DNA nanostructures in living cells. ACS Appl. Mater. Interfaces 2021; 13: 46375.
- Frtús A, Smolková B, Uzhytchak M, Lunova M, Jirsa M, Kubinová Š, Dejneka A, Lunov O. Analyzing the mechanisms of iron oxide nanoparticles interactions with cells: A road from failure to success in clinical applications. J Control Release 2020; 328: 59.
- Levada K, Pshenichnikov S, Omelyanchik A, Rodionova V, Nikitin A, Savchenko A, Schetinin I, Zhukov D, Abakumov M, Majouga A, Lunova M, Jirsa M, Smolková B, Uzhytchak M, Dejneka A, Lunov O. Progressive lysosomal membrane permeabilization induced by iron oxide nanoparticles drives hepatic cell autophagy and apoptosis. Nano Converg. 2020; 7: 17.