Open access
Thanks to the involvement of the laboratory in large research infrastructures Czech-BioImaging and EuroBioImaging, its technologies and services are open to any internal or external researcher with a justified research aim and a feasible experimental plan, as long as the necessary resources (instruments, animal house, staff) are available. The implemented open-access procedures described below should help the users find useful and feasible goals and ensure that all requirements set by the Czech law, infrastructure and institutional bylaws, the laboratory rules and the technical parameters are met.
General policies
The ISI-MR facility rules are based on Acts 130/2002 Coll. (public support of R&D), 341/2005 Coll. (public research institutions), 246/1992 Coll. and related (protecting animals from cruelty), 89/2012 Coll. (civil code), 121/2000 Coll. (copyright law), 262/2006 (labour code), European Commission communication 2014/C 198/01 (framework for state aid for R&D and innovation), the bylaws of the Ministry of Education, Youth and Sports of the Czech Republic applicable to large research infrastructure Czech-BioImaging, and the bylaws of the ISI. Proper implementation of all applicable requirements is believed to be achieved with the following rules:
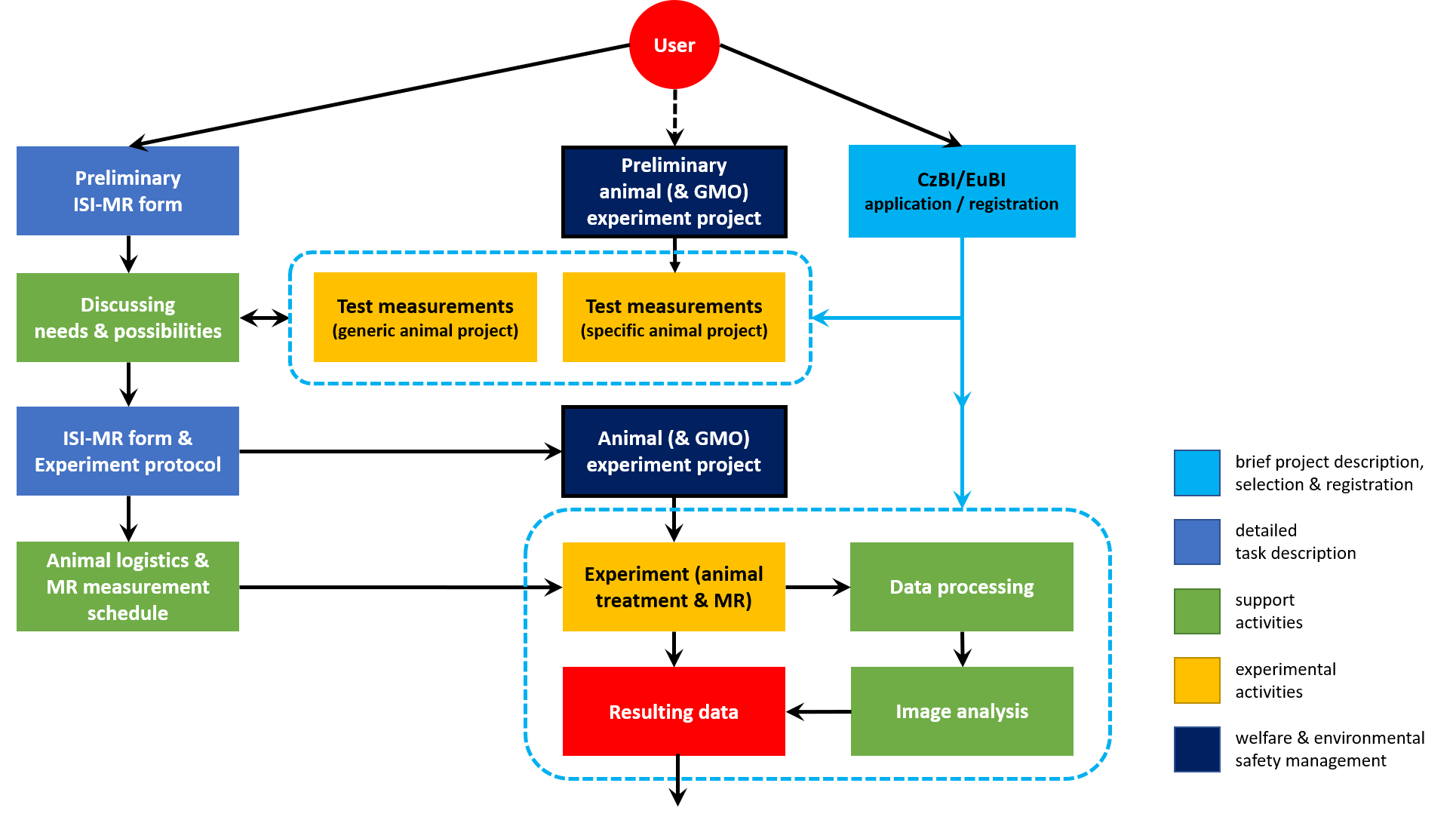
• Services and measurements are based on individual projects – a project is considered to be a set of measurements or complementary services realized for the project researcher (applicant) and his/her team in a particular time period, with a specific goal and typically also defined devices, methods and parameters. The experimental project does not have to correspond with a grant project or any other externally defined activity.
• To be granted open access with Czech-BioImaging support, each external applicant is first required to fill in a request form on the Czech-BioImaging open access web page, or find an analogical service at the Euro-BioImaging web. Each application is assessed by the infrastructure organs from the point of view of its R&D potential, feasibility, resources availability and economic parameters. The applicant will be notified of the result within 30 days.
• To be granted open access without public support, the external applicant is advised to consult the ISI-MR facility at czbi@isibrno.cz and proceed as follows.
• In most cases, additional information will be required from the applicant in order that the technical, legal, logistics and funding details may be specified. Specifically, applicants planning to study animals in vivo should fill in the animal use form, those planning to administer experimental substances to animals (e.g., for MR contrast modification) should fill in the substance administration form, and those involving inanimate samples (ex vivo tissues, phantoms etc.) should submit the inanimate sample form. Filling in and submitting these forms is recommended already before the initial application.
• Should the applicant find the default data-management terms, as described in the Data Management section, unsuitable or insufficient to properly handle the intellectual property and data ownership and access rights for the intended project, an individual agreement would have to be signed by qualified persons of the ISI and the legal entity on whose behalf the applicant acts.
• Once agreement is reached on the content and time plan of the experiment, a written memorandum will be signed by the qualified researchers of the ISI-MR facility and the user; this memorandum can be later amended in the same way.
• As a rule, the open access is provided as a complex service in which the user is not directly executing the experiment at the ISI-MR facility premises, the reasons including the necessary technical and laboratory-specific competence, animal use legislation, safety regulations, health and liability insurance issues etc. Nevetheless, personal participation of the user is recommended at least in the decisive phases of the experiment.
• Small-scale pilot experiments for the verification of the technical possibilities of the devices and protocols may be arranged in a simplified procedure. If the pilot experiments or previous experience indicate the need for customized procedures, their development may be the subject of collaborative research involving the user and both the ISI-MR facility and the ISI MR Research Group.
• The ISI-MR facility cooperates with the ISI MR Research Group on the development of higher-quality methods for the benefit of future ISI-MR facility users. These works do not reduce the facility capacity dedicated to public service and their direct costs are paid by specific research grants whenever possible.
• The user is expected to partially compensate the service cost. The specific requirements will be determined based on the task specification, as described in the forms mentioned above. Please contact us at czbi@isibrno.cz for more information.
Infrastructure-subsidized open access flow chart
Commercial open access flow chart